Work programme
transMed joins four academic institutions and four non-academic and SME partners, with one academic clinical research partner organisation and four more SME partner organisations. All partners add specific knowledge on retinal drug development and collaborations are already ongoing. This ensures the highest level of specific expertise in research, also providing the transMed ESRs with an exceptional overview of all stages of translational research together with insights into the biotech industry.
transMed focuses on the development of novel treatments for RD, a major unmet medical need. While previous research already provided many exciting perspectives, major challenges remain:
- therapeutic targets, their definition/refinement and compound development;
- biomarkers for disease diagnostics and treatment validation;
- drug delivery systems (DDS) to bypass or overcome the blood-retinal-barrier;
- the chronic nature of RD, which requires DDS that allow controlled, long-term release with as long as possible treatment intervals.
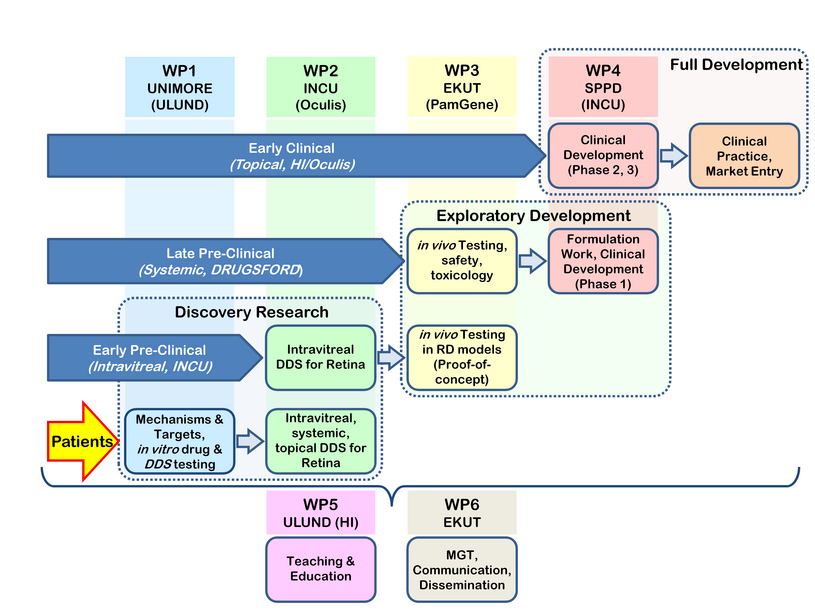
To address these challenges, transMed uses a stair-wise approach as shown in Figure 2: Specifically, the INCU DDS, which is in early pre-clinical development, is very innovative and has promising physicochemical properties for intravitreal drug application. The compounds and DDS for systemic delivery developed in the DRUGSFORD project are already in the final stages of pre-clinical development, and may enter phase 1/2 trials during the transMed project. Finally, the DDS pioneered by HI and Oculis for topical delivery to the retina is now in phase 1 clinical trials and may move forward to Phase 2 within the next four years. Thus, already from this parallel line-up of partners and projects, the ESRs will get a broad overview of translational research. transMed focuses in particular on the critical transition from pre-clinical to clinical development (i.e. exploratory development), such as pre-clinical animal studies and regulatory aspects including GCP in phase 1-3 clinical studies (SPPD, Oculis, HCTC).

